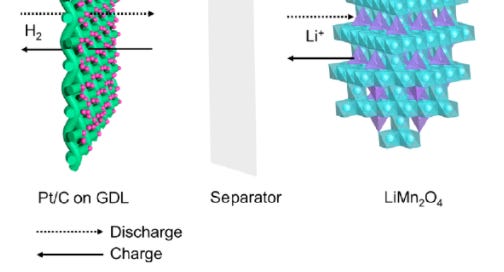
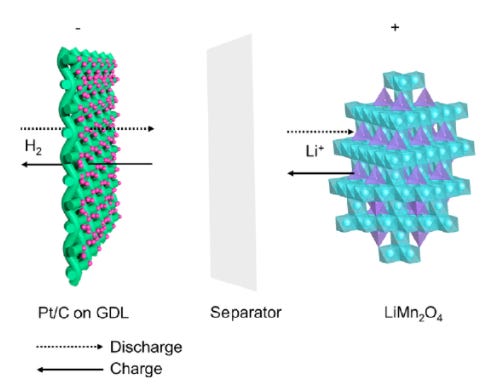
Batteries come in all shapes, sizes, and chemistries. For this issue I will highlight the novel lithium manganese oxide hydrogen battery (LMnO-H).
Grid scale energy storage is a hot topic now as the country and world considers incorporating batteries into the power distribution system. We highlighted the vanadium flow battery as a solution that is being used in countries throughout the world.
The LMnO-H battery proposed by researchers at the University of Science and Technology of China is a new concept targeted at improving grid storage. The battery couples electrocatalytic hydrogen gas at the anode with a lithium metal oxide at the cathode. It takes advantage of hydrogen evolution and oxidation reactions (HER and HOR) that occur quickly and are highly stable. Paired with an LMnO cathode it showed reasonable energy density, charge/discharge rates, and cycle life.
The Cell
Effectively a hybrid between a fuel cell and lithium ion battery, an LMnO cathode is paired with hydrogen gas anode in an aqueous lithium sulfate electrolyte.
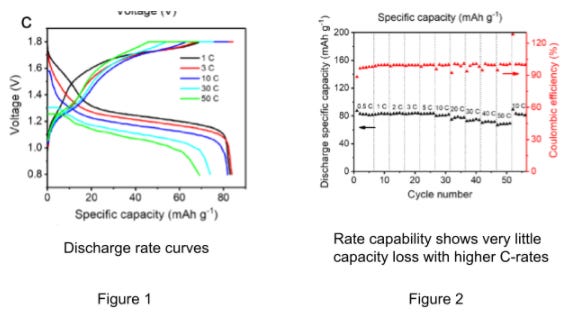
Under electric load, the cell exhibits a discharge voltage of 1.3V and good capacity. The cell yielded 83mAh/g at 1C and 69.1mAh/g at 50C with long cycle life. The mass loading of the LMnO electrode was 3 mg/cm2. The rate capability up to 50C is good with a 50C/1C capacity ratio of .83. See figures 1 and 2 below for graphs.
The half cell and full reaction is shown below. The cathode active material is reduced and the anode is oxidized yielding water as a byproduct.
How it Works?
During charge lithium is extracted from the LMnO cathode into the aqueous electrolyte (lithium sulfate). Simultaneously hydrogen gas is generated at the anode.
During discharge Li+ in the electrolyte is inserted back into the LMnO (Lithium Manganate) spinel structure and hydrogen gas is absorbed and oxidized on the anode due to the highly active hydrogen oxidation reaction initiated by the platinum/carbon catalyst.
Electrochemical Performance Metrics
Traditional LMnO-C batteries have long struggled with high charge/discharge rate abilities, and the authors validated this. The LMnO-C cell uses traditional graphite at the anode instead of hydrogen. As you can see in the figure below, both rate capability testing and 5C discharge testing show clear limitations for the LMnO-C cell.

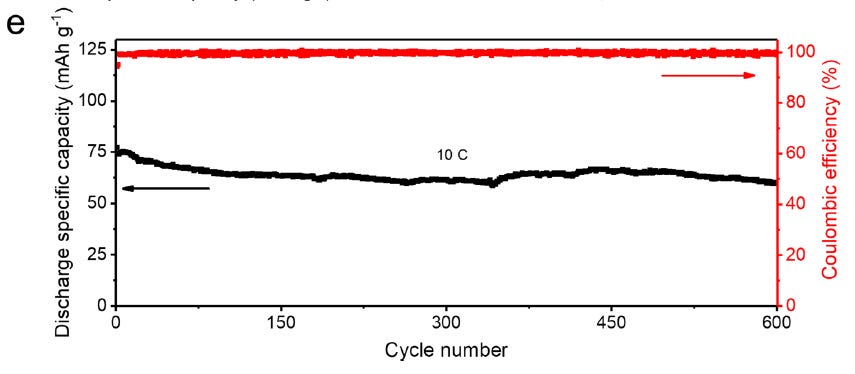
An LMnO-H cell (3mg/cm2) shows good performance with a 2C constant current charge and discharge current of 10C. The cells were charged to 1.8V.
With a 10C discharge the cell retains ~80% capacity after 600 cycles.
Most cells cycle well at low mass loadings because of minimal resistance in thin films. The authors also evaluated cells at mass loadings of 9.5 mg/cm2, roughly three times higher. As you can see, the achievable capacity when comparing loadings of 1.5mg/cm2 and 9.5mg/cm2 is about 40% lower for the thicker electrode.
The performance of this cell is actually quite good for hydrogen coupled batteries, and is far superior than the ones referenced below.
Conclusions
Grid storage needs cell chemistries that can charge/discharge extremely quickly to supply power in times of need. This battery which is essentially a hybrid between a fuel cell and a lithium ion battery yields fairly good performance.
But the question comes down to practicality. If this chemistry is being targeted for grid storage, it seems like the vanadium flow battery is a more viable option. The engineering to design the LMnO-H battery at scale requires laying piping for hydrogen gas, which could be a costly investment. On the other hand, the vanadium flow battery uses liquid anolyte and catholyte which could be delivered to holding tanks by a tanker truck.
This is a unique application for a lithium ion battery, but more data is needed on long term cycling and cost implications. I welcome the #battchat and #batterytwitter community to their thoughts around the viability of this particular chemistry and application.
References:
A High-Rate Lithium Manganese Oxide-Hydrogen Battery - Z. Zhu, M Wang. April 17th, 2020. Nano Letters https://pubs.acs.org/doi/10.1021/acs.nanolett.0c00044.
Supplementary Information for reference 1. https://pubs.acs.org/doi/suppl/10.1021/acs.nanolett.0c00044/suppl_file/nl0c00044_si_001.pdf