A few months ago I shared a deep dive on lithium sulfur batteries and the challenges of commercializing the technology. During the time of posting in June, news was released that one of the leaders in the industry, Oxis Energy, was insolvent and was not going to be able to move forward with their technology.
This troubling news was a big shot in the gut for Li-S proponents given Oxis’ 20+ years of experience in the industry. Oxis Energy announced that some 200 of their patents would be up for sale, and recent reports show that Johnson Matthey purchased them along with other assets for accelerating their push toward producing green hydrogen.
A Newcomer Emerges
A dose of negativity is quickly followed up with positivity as last week a new contender, Lyten, emerged with a renewed confidence in lithium sulfur technology for electric vehicle applications.
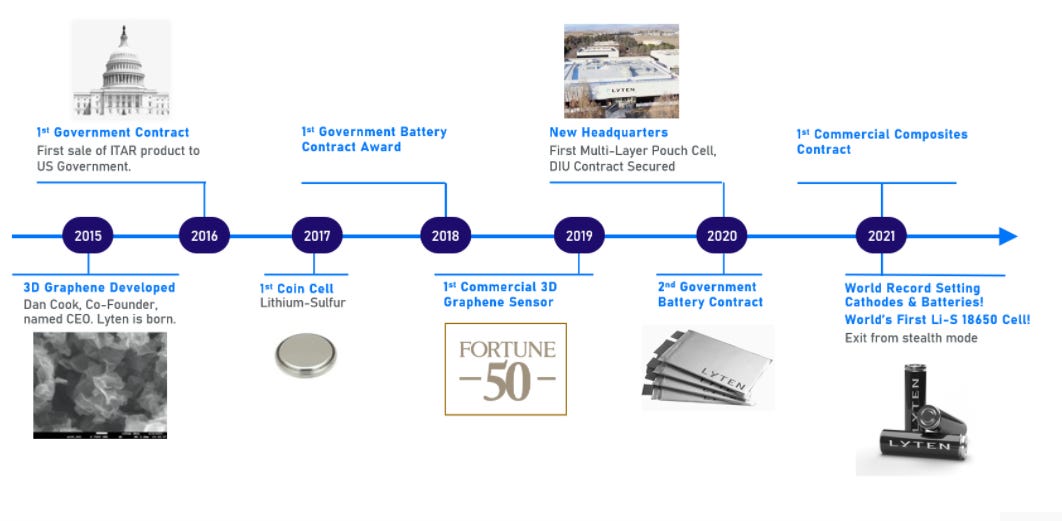
The privately held firm was founded in 2015 with the development of 3D Graphene. This has led to a variety of milestones laid out in the timeline below with a few government contracts giving credence to the patented technology.
Lyten claims that their Li-S battery technology is ready for pouch, prismatic, and cylindrical form factors, while also disclosing other performance specifications.
3x specific energy density compared to current Li-ion cells. (*if we estimate today’s Li-ion cells to ~250-300Wh/kg Lyten’s could be ~750 Wh/kg)
1000 cycles with Lyten Li-S batteries
No combustion when punctured, crushed, or overcharged.
60% lower carbon footprint than conventional Li-ion
No Ni, Mn, or Co in cathode
Supply chain for its materials are <1000 miles away compared to industry average of 29,000 miles.
>250 patents granted and pending.
Reliable power from -30C - +60C
Addressing the Challenge of Li-S
As previously mentioned, one of the big challenges of Li-S batteries is mitigating the shuttling of sulfur into the electrolyte. When discharged, sulfur is reduced to lithium polysulfide (Li2S8) and further reduction to Li2S4 causing a significant increase in the concentration/viscosity of polysulfides. Li2S4 is further reduced to insoluble Li2S and Li2S2 at 100% discharge. The charging process oxidizes Li2S and Li2S2 to polysulfides until being finally converted back to elemental sulfur.
The evolution of polysulfides impacts the electrochemical performance of the cell and is a big driver in preventing the mass adoption of Li-S technologies.
Anchoring Mechanisms
If researchers could mitigate the dissolution of sulfur into polysulfides one could theoretically make a cell with reasonable cycling performance. This paper which was kindly shared by Frank Wunderlich-Pfeiffer (@FrankWunderli13) on Twitter, shares physical and chemical anchoring strategies for Li-S batteries.
Physical confinement involves using sulfur host materials that impede polysulfide migration. Lyten claims to use a novel 3D Graphene® that “cages” sulfur effectively to enable improved performance. One could imagine sulfur being sandwiched in amongst the pores of graphene preventing it from escaping.
Chemical confinement strategies involve polar-polar interactions, Lewis acid-base interactions, redox reactions, and covalent binding interactions. These would involve material additives, binders, and metal oxides that could trap sulfur. See pages 6-9 in this paper for more information.
My Lyten Take
In section 4.2 of the research paper the authors highlight a synthesized “N-doped graphene (rNGO) with a 3D porous framework structure.” Some cycling data is shared in this paper suggesting that the rNGO/S battery can successfully cycle 1000 cycles. Although the cell specific capacity at 1000 cycles is <80% of it’s initial value, at 500-600 Wh/kg it is still still significantly higher than a traditional Li-ion battery.
Has Lyten cracked the code on Li-S? The cycling data provided in the research paper referenced above does not provide all the answers, but it suggests that it is possible. Could 3D Graphene be used on both the anode and cathode? Are there other anchoring additives being utilized? There are obviously lots of specifics that we need to truly know if the batteries are ready for commercialization. With the pilot line scheduled for completion in Dec 2021 in San Jose, CA we will have a better idea if they can produce quality cells!
References:
2) Host Materials Anchoring Polysulfides in Li-S Batteries Reviewed. L. Zhou, D. Danilov, and others. June 2020
4) Ternary Solvent Package and 2-Mercaptobenzothiazole for lithium-sulfur batteries.